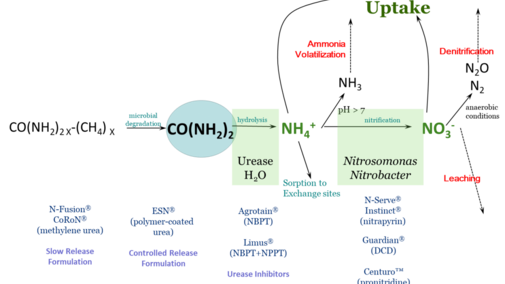
Nitrogen fertilizer management is challenging due to the many factors that influence fertilizer nitrogen (N) after it is applied. Of primary concern is the potential for fertilizer N to be lost to the environment and thus unavailable to the crop – either through ammonia volatilization, denitrification, or leaching. Fertilizer N is most often in three chemical forms: ammonium (NH4+), nitrate (NO3) or urea.
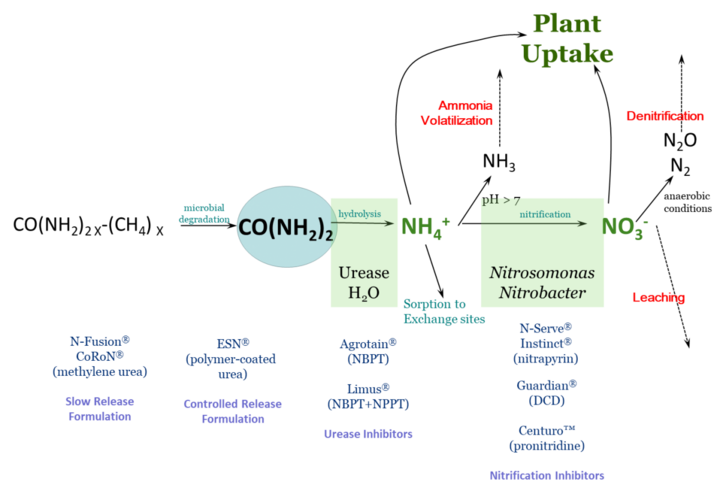
Figure 1 schematically describes N loss reactions that can occur in the soil, as well as management steps that can be taken to protect against N loss. This article focuses on inhibitors – either of urease activity or of nitrification. These compounds are sometimes called stabilizers, or the N fertilizer protected by them as stabilized N, though this can be confusing terminology.
Urease Inhibitors
These chemical compounds block the activity of the enzyme urease. Urease is found in soil as well as in plant residues. Urease, along with water, will hydrolyze, or break down, urea into ammonium. The loss process that urease inhibitors protect against is ammonia volatilization. With high pH soil, or soil/residue environments that are poorly buffered against an increase in pH, the rapid hydrolysis of urea will result in an accumulation of ammonia (NH3) rather than ammonium.
Ammonia can be lost to the atmosphere as a gas. Under worst-case conditions, this loss can be rapid and significant. Research studies have found greater than 50% of applied fertilizer N lost to the atmosphere under these conditions. By keeping urea from hydrolyzing, urease inhibitors protect against ammonia volatilization, keeping fertilizer N in the urea form.
Since urea is very water soluble, such inhibition allows time for rainfall or irrigation to move urea into the soil. Once incorporated into the soil, to a depth of just 2-3 inches, urea-N is protected from ammonia volatilization, because ammonium that is released through hydrolysis is quickly held on soil cation exchange sites. Urease inhibitors break down with time, but typically can protect against ammonia loss from surface applied fertilizers for a couple of weeks, depending on temperature and moisture conditions.
Products with known efficacy for inhibiting urease activity are N-(n-butyl) thiophosphoric triamide (NBPT) and N-(n-propyl) thiophosphoric triamide (NPPT). These active ingredients are found in products with tradenames of Agrotain™ (NBPT) and Limus™ (NBPT & NPPT). There are also other products that contain NBPT, since it is no longer patent-protected.
Nitrification Inhibitors
These chemical compounds temporarily reduce populations of Nitrosomonas and Nitrobacter bacteria in soil. These are the bacteria responsible for converting ammonium to nitrite (Nitrosomonas) and nitrite to nitrate (Nitrobacter). These compounds protect against both denitrification and leaching by retaining fertilizer N in the ammonium form. Ammonium (NH4+) is a positively charged ion (cation) which can be held on negatively charged exchange sites in soil – present in clays and organic matter. This negatively charged anion nitrate (NO3-) can be converted to N2O or N2 gases with anaerobic (waterlogged) conditions, or can leach below the root zone with rain with well-drained soils.
Products with known efficacy for inhibiting nitrification are dicyandiamide (DCD), nitrapyrin, and pronitradine. Nitrapyrin has long been sold as N-Serve™ and Instinct™, and pronitradine has recently come into the market with the tradename Centuro™. Nitrapyrin and DCD are not patent protected, and may be found in a variety of products. Nitrification inhibitors, like urease inhibitors, will break down over time and will no longer be effective. The rate of breakdown is influenced particularly by temperature, so these products generally remain effective longer at cooler soil temperatures, with efficacy ranging from two to several weeks.
Efficacy
The University of Nebraska-Lincoln has research experience with all the active ingredients and products listed above. We find these generally perform as advertised in inhibiting either urease activity or nitrification. However, such protection may not translate into yield or profit benefits. If weather and soil conditions are not conducive to N loss, there is no benefit to protection against loss with the use of inhibitors. If N rates are excessive, N loss can occur but have no impact on yield. With excessive N rates, there will be no benefit from the use of inhibitors.
Challenges
There are a variety of products on the market today claiming to be N stabilizers, increasing yield or profit. If an active ingredient is not listed above, the University of Nebraska-Lincoln either has no research experience with the product, or we have found the product to be ineffective in our research studies.
A growing challenge is the number of products on the market which contain the active ingredients listed above, but at lower concentrations, or different formulations, from products we have evaluated in our research. Thus, a product may contain nitrapyrin as a nitrification inhibitor, or NBPT as a urease inhibitor, but UNL has no research experience with it. The large number of products on the market today makes it impossible to evaluate each product formulation. In such cases, we encourage growers to fully understand the products they choose, and understand their mode of action and the N loss process they are intended to protect against.